Strategic Outsourcing of NHP Studies to Specialized CROs
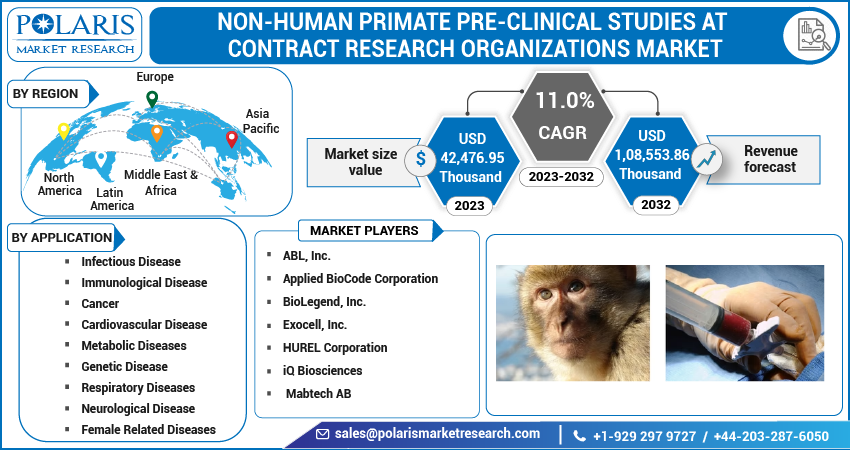
The global Non-Human Primate (NHP) pre-clinical studies at Contract Research Organizations (CROs) market is poised for remarkable growth over the next decade. Driven by the escalating demand for translational research, stringent drug development protocols, and increased outsourcing of preclinical activities by pharmaceutical and biotechnology companies, the market is emerging as a vital segment in the preclinical research ecosystem.
In 2024, the market was valued at approximately USD 1,08,553.86 thousands by 2032, and it is projected to grow at a CAGR of 11.0% from 2025 to 2034. NHPs such as macaques and marmosets have physiological and immunological characteristics closely resembling humans, making them indispensable models for testing the safety and efficacy of biologics, gene therapies, vaccines, and small molecule drugs.
The increasing prevalence of chronic diseases, emergence of novel drug candidates, and accelerated regulatory scrutiny for human trials have led to heightened reliance on preclinical CRO services, especially those offering NHP studies. Moreover, rising R&D investments and the focus on reducing clinical failure rates are amplifying the demand for in vivo toxicology studies in NHP models.
Market Segmentation
The Non-Human Primate Pre-clinical Studies at CROs Market is segmented by study type, application, and end-user.
By Study Type:
- Toxicology Studies
- Single-dose toxicity
- Repeat-dose toxicity
- Reproductive toxicity
Toxicology studies account for the largest market share, primarily due to regulatory requirements for safety profiling before Investigational New Drug (IND) applications. However, disease modeling and vaccine testing are gaining momentum, driven by increasing biopharmaceutical R&D focused on infectious diseases and immunotherapies.
By Application:
- Oncology
- Neurology
- Infectious Diseases
- Cardiovascular Diseases
- Immunology
- Others (Metabolic, Genetic Disorders)
The oncology segment dominates due to the surge in novel cancer therapeutics, including immuno-oncology and cell-based therapies. NHPs are widely used in translational research services for modeling complex tumor microenvironments and immune responses.
By End-User:
- Pharmaceutical and Biopharmaceutical Companies
- Academic and Research Institutes
- Government Agencies
Pharmaceutical and biotech companies represent the primary clientele for preclinical CRO services, leveraging external NHP expertise to streamline drug development timelines and comply with regulatory norms.
Regional Analysis
North America
North America leads the global market, accounting for the largest share in 2024. The U.S., in particular, has a stronghold due to its advanced biomedical infrastructure, high R&D expenditure, and presence of leading CROs specializing in non-human primate models. The FDA’s regulatory emphasis on comprehensive preclinical data further propels the demand.
Moreover, several high-profile biopharma collaborations with CROs offering NHP capabilities have emerged post-pandemic, primarily for monoclonal antibodies, gene therapy, and vaccine research.
Europe
Europe holds the second-largest market share, with the UK, Germany, and France emerging as key countries. The growing prevalence of rare diseases and support from the European Medicines Agency (EMA) for early-stage drug evaluation is bolstering market activity. European CROs increasingly focus on ethical animal use protocols and 3Rs (Replacement, Reduction, Refinement) compliance, which enhances the credibility and appeal of their in vivo toxicology studies.
Asia Pacific
The Asia Pacific region is witnessing the fastest growth, fueled by rising biomedical investments, favorable government policies, and cost advantages for outsourcing. Countries like China and India are becoming global hubs for preclinical CRO services, including NHP studies. China’s expanding biotech ecosystem and India’s skilled scientific workforce have attracted multinational pharmaceutical clients.
Additionally, regulatory reforms and government-backed biotech parks in South Korea and Singapore are paving the way for CRO expansion with advanced translational research services involving non-human primates.
Latin America
In Latin America, Brazil and Mexico are the major contributors. While still an emerging market, increased clinical research activities and collaborations with U.S.-based CROs have encouraged the uptake of NHP-based studies. Budget constraints and ethical concerns remain challenges, but investments in life sciences infrastructure are gradually improving the landscape.
Middle East & Africa
The MEA region remains a nascent market, limited by regulatory and infrastructural challenges. However, growing interest in biosimilar development, particularly in the Gulf Cooperation Council (GCC) countries, is likely to open niche opportunities for preclinical CROs offering non-human primate models. South Africa has shown interest in vaccine development and infectious disease studies, which could include greater NHP usage in the future.
Challenges
Despite its growth potential, the NHP preclinical CRO market faces several challenges:
- Limited NHP Supply: Ethical sourcing and breeding of primates are strictly regulated, limiting availability.
- High Study Costs: NHP studies are significantly more expensive than rodent-based models, limiting their use to late preclinical stages.
- Public and Regulatory Scrutiny: The use of primates in research is a sensitive issue, with increasing advocacy for alternative models.
- Infrastructural Requirements: Setting up and maintaining NHP research facilities requires high capital investment and stringent biosecurity measures.
Conclusion
The Non-Human Primate Pre-clinical Studies at Contract Research Organizations market is emerging as a critical component in the global drug development chain. With the increasing complexity of biologic and gene therapy pipelines, the demand for non-human primate models is expected to grow significantly. From oncology and neurology to vaccine development, NHP studies conducted by specialized CROs are providing crucial translational insights.
Backed by advanced technology, stringent regulatory compliance, and growing industry collaborations, CROs are well-positioned to meet the evolving demands of the biopharmaceutical sector. As ethical practices and scientific advancements converge, the market promises not only expansion but also transformation in how preclinical research shapes the future of global healthcare innovation.
More Trending Latest Reports By Polaris Market Research:
Point of Care (PoC) Diagnostics Market
Next Generation Sequencing (NGS) Market
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Oyunlar
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- Travels

